Class 11 Chemistry Notes - Unit 4: Classification of Elements and Periodic Table
Class 11 Chemistry Notes - Unit 4: Classification of Elements and Periodic Table
Target Audience: Class 11 Students, Nepal (NEB Curriculum)
Teaching Hours: 5
Objective: Understand the modern periodic table, element classification, and periodic trends in atomic properties.
Key Points
- Modern periodic law organizes elements by increasing atomic number.
- Elements are classified into groups, periods, and blocks (s, p, d, f).
- Effective nuclear charge influences periodic properties.
- Periodic trends include atomic radii, ionization energy, electronegativity, and metallic character.
Detailed Notes: Unit 4 - Classification of Elements and Periodic Table
These notes are designed for Class 11 students in Nepal, aligned with the NEB curriculum, covering the periodic table and its trends with clear explanations and diagrams.
4.1 Modern Periodic Law and Modern Periodic Table
Modern Periodic Law: Proposed by Henry Moseley, states that the physical and chemical properties of elements are periodic functions of their atomic numbers.
Modern Periodic Table: Arranges elements in order of increasing atomic number in:
- Periods: 7 horizontal rows (1 to 7), based on principal quantum number (n).
- Groups: 18 vertical columns, elements with similar valence electron configurations.
Diagram:
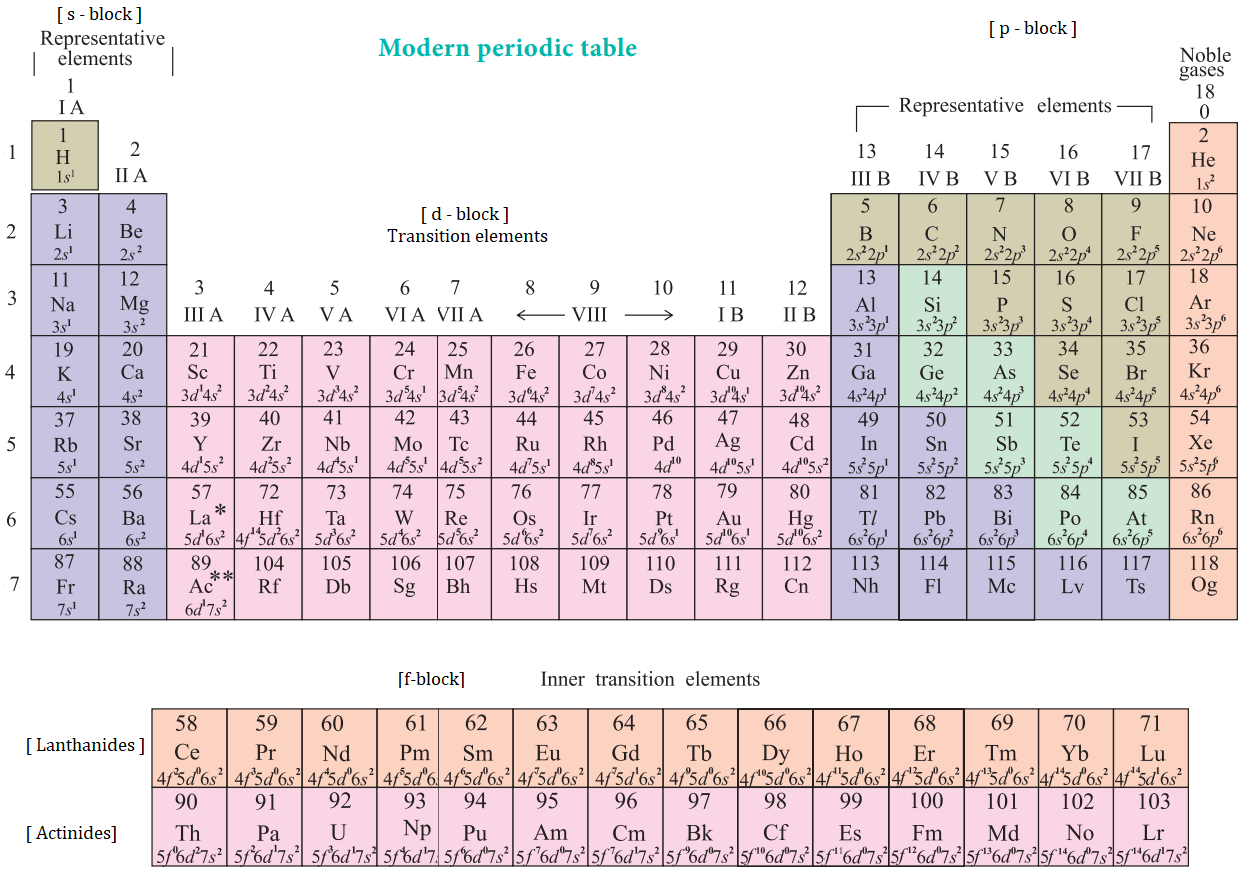
Figure 1: Modern periodic table showing groups, periods, and blocks.
Note: Add an image named modern_periodic_table.png depicting the periodic table with labeled groups (1-18), periods (1-7), and blocks (s, p, d, f).
4.2 Classification of Elements into Different Groups, Periods, and Blocks
Classification:
- Groups: 18 groups; Group 1 (alkali metals), Group 2 (alkaline earth metals), Group 17 (halogens), Group 18 (noble gases).
- Periods: Period 1 (2 elements), Periods 2-3 (8 elements), Periods 4-5 (18 elements), Periods 6-7 (32 elements).
- Blocks: Based on subshell filled by valence electrons:
- s-block: Groups 1, 2 (e.g., Na: [Ne]3s¹).
- p-block: Groups 13-18 (e.g., Cl: [Ne]3s²3p⁵).
- d-block: Groups 3-12 (transition metals, e.g., Fe: [Ar]3d⁶4s²).
- f-block: Lanthanides and actinides (inner transition metals).
Example: Oxygen (Z=8) is in Period 2, Group 16, p-block ([He]2s²2p⁴).
4.3 IUPAC Classification of Elements
IUPAC Nomenclature: Elements are named systematically, especially for superheavy elements (Z > 100).
- Elements Z=101 to 118 use Latin/Greek roots for digits: 0 (nil), 1 (un), 2 (bi), etc., with suffix
-ium. - Example: Z=112 (Copernicium, Cn) was temporarily Ununbium (un-un-bi-ium).
Purpose: Standardizes naming for newly discovered elements.
4.4 Nuclear Charge and Effective Nuclear Charge
Nuclear Charge: Total positive charge of the nucleus, equal to the atomic number (Z × e, where e is proton charge).
Effective Nuclear Charge (Zeff): Net positive charge experienced by valence electrons, reduced by shielding effect of inner electrons.
Zeff = Z - S, where S is screening constant.
Trend:
- Increases across a period (more protons, similar shielding).
- Decreases down a group (more shells, increased shielding).
Example: For Na (Z=11), Zeff is lower than for Cl (Z=17) in Period 3 due to less nuclear charge and more shielding.
4.5 Periodic Trend and Periodicity
Periodicity: Regular variation in element properties across periods and groups due to electron configurations.
Diagram:
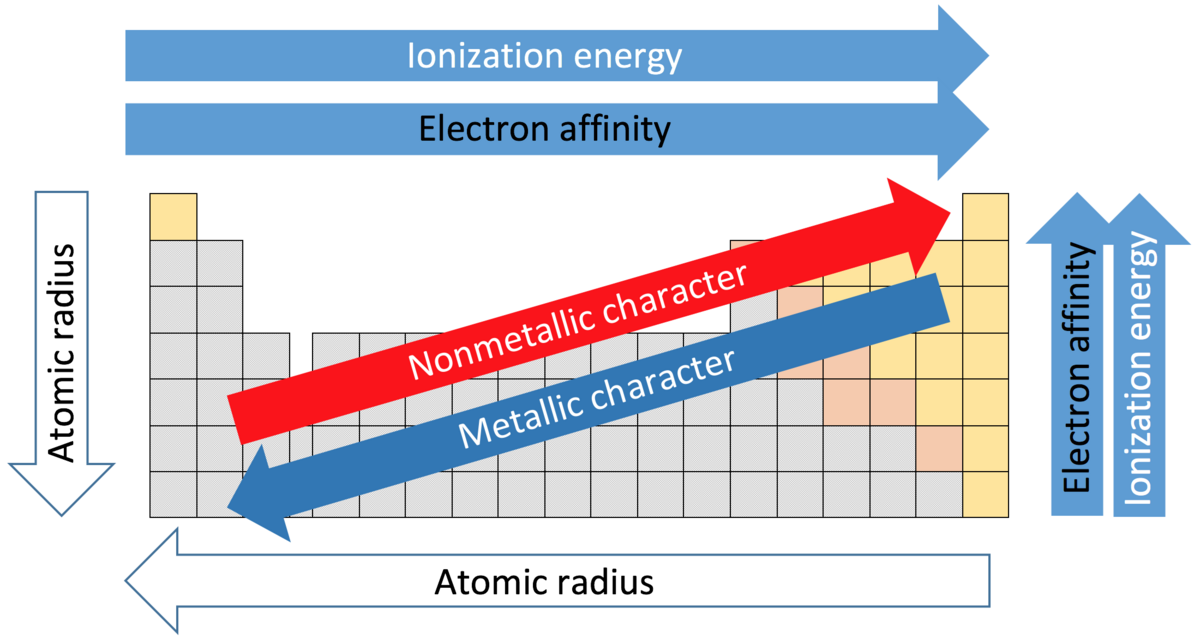
Figure 2: Graph showing trends in atomic radii, ionization energy, and electronegativity.
Atomic Radii
Definition: Half the distance between nuclei of two identical atoms covalently bonded.
Trend:
- Across a Period: Decreases due to increased Zeff, pulling electrons closer.
- Down a Group: Increases due to additional electron shells.
Example: Li (152 pm) > Na (186 pm); F (72 pm) < O (74 pm).
Ionic Radii
Definition: Radius of an ion.
Trend:
- Cations: Smaller than neutral atoms (e.g., Na⁺ < Na) due to electron loss and higher Zeff.
- Anions: Larger than neutral atoms (e.g., Cl⁻ > Cl) due to electron gain and reduced Zeff.
- Similar to atomic radii: Decreases across period, increases down group.
Example: Na⁺ (102 pm) < Na (186 pm); Cl⁻ (181 pm) > Cl (99 pm).
Ionization Energy
Definition: Energy required to remove the outermost electron from a gaseous atom.
Trend:
- Across a Period: Increases due to higher Zeff and smaller size.
- Down a Group: Decreases due to larger size and shielding.
Example: Li (520 kJ/mol) < Ne (2081 kJ/mol); Na (496 kJ/mol) < Li.
Electron Affinity
Definition: Energy change when an electron is added to a gaseous atom.
Trend:
- Across a Period: Increases (more negative) due to higher Zeff.
- Down a Group: Decreases (less negative) due to larger size.
Example: Cl (-349 kJ/mol) > F (-328 kJ/mol); Cl > Br (-325 kJ/mol).
Electronegativity
Definition: Ability of an atom to attract shared electrons in a bond (Pauling scale).
Trend:
- Across a Period: Increases due to higher Zeff.
- Down a Group: Decreases due to larger size.
Example: F (4.0) > O (3.5); F > Cl (3.0).
Metallic Character
Definition: Tendency to lose electrons and form positive ions.
Trend:
- Across a Period: Decreases as elements become less metallic (metals to non-metals).
- Down a Group: Increases due to easier electron loss.
Example: Na (metal) > Si (metalloid) > Cl (non-metal); Cs > Na.
Summary Table: Periodic Trends
| Property | Across Period | Down Group |
|---|---|---|
| Atomic Radii | Decreases | Increases |
| Ionic Radii | Decreases (cations/anions) | Increases |
| Ionization Energy | Increases | Decreases |
| Electron Affinity | Increases (more negative) | Decreases (less negative) |
| Electronegativity | Increases | Decreases |
| Metallic Character | Decreases | Increases |