Unit 9.3: Halogens (5 Teaching Hours)
-
General Characteristics of Halogens
- Group 17 Elements
- Halogens (F, Cl, Br, I, At) have seven valence electrons (ns²np⁵), needing one electron for stable octet.
- Exist as diatomic molecules (Cl₂, Br₂, I₂). Physical state: F₂, Cl₂ (gases); Br₂ (liquid); I₂ (solid) at room temperature.
- Electronegativity decreases down the group (F > Cl > Br > I). Strong oxidizing agents; reactivity decreases (F most reactive, I least).
- Form ionic/covalent compounds with metals/non-metals.
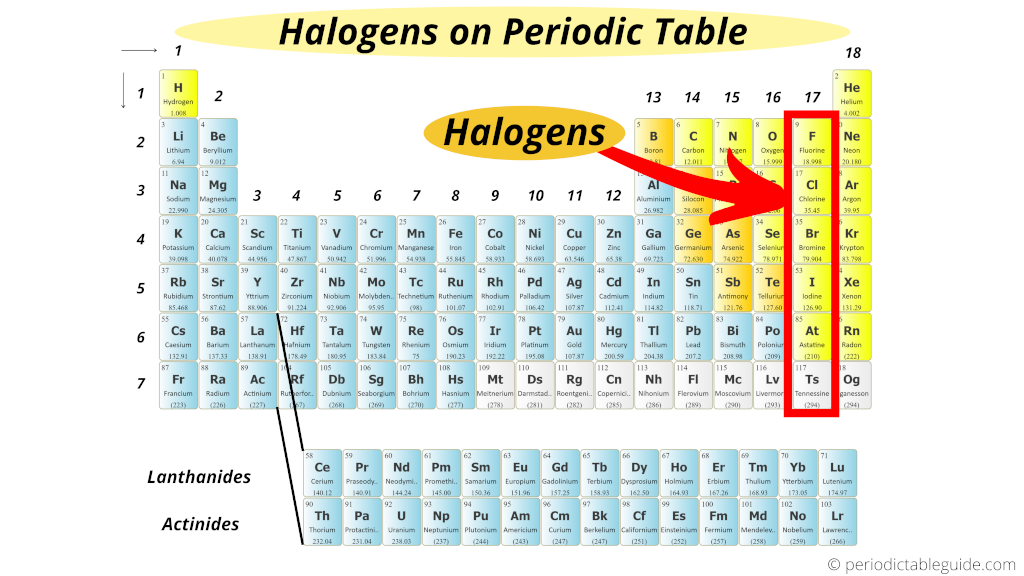
- Figure 1: Periodic Trends in Halogens (Diagram showing trends in electronegativity, atomic size, and reactivity).
- Group 17 Elements
-
Comparative Study on Preparation of Halogens (Cl₂, Br₂, I₂)
- Preparation
- No diagrams or descriptions required as per curriculum.
- Chlorine (Cl₂): Prepared by oxidation of chloride ions (e.g., heating NaCl with MnO₂ and conc. H₂SO₄).
- Bromine (Br₂): Prepared by oxidation of bromide ions (e.g., heating NaBr with MnO₂ and conc. H₂SO₄).
- Iodine (I₂): Prepared by oxidation of iodide ions (e.g., heating NaI with MnO₂ and conc. H₂SO₄ or using Cl₂ to displace I₂ from KI).
- Preparation
-
Chemical Properties of Halogens (Cl₂, Br₂, I₂)
- With Water
- Cl₂: Disproportionates to form HCl and HClO: Cl₂ + H₂O → HCl + HClO.
- Br₂: Less reactive, forms HBr and HBrO to a lesser extent.
- I₂: Sparingly soluble, does not react significantly with water.
- With Alkali
- Cl₂: Cold dilute NaOH forms hypochlorite: Cl₂ + 2NaOH → NaCl + NaOCl + H₂O. Hot conc. NaOH forms chlorate: 3Cl₂ + 6NaOH → 5NaCl + NaClO₃ + 3H₂O.
- Br₂: Similar, forms NaBr, NaOBr (cold), or NaBrO₃ (hot).
- I₂: Forms NaI, NaIO (cold), or NaIO₃ (hot), less reactive.
- With Ammonia
- Cl₂: Forms nitrogen trichloride (explosive): 8NH₃ + 3Cl₂ → 6NH₄Cl + NCl₃.
- Br₂: Forms nitrogen and ammonium bromide: 8NH₃ + 3Br₂ → 6NH₄Br + N₂.
- I₂: Forms nitrogen triiodide (explosive when dry): 5NH₃ + 3I₂ → 3NH₄I + NI₃.
- Oxidizing Character
- Halogens oxidize other substances; strength decreases down the group (Cl₂ > Br₂ > I₂).
- Cl₂ oxidizes Fe²⁺ to Fe³⁺, Br⁻ to Br₂, I⁻ to I₂.
- Br₂ oxidizes I⁻ to I₂ but cannot oxidize Cl⁻.
- I₂ is the weakest oxidizer.
- Bleaching Action
- Cl₂: Bleaches by oxidation (nascent oxygen via HClO): Cl₂ + H₂O → 2HCl + [O]. Permanent bleaching (e.g., textiles, paper).
- Br₂: Weaker bleaching action, less effective.
- I₂: No significant bleaching action.
- Figure 2: Bleaching Action of Chlorine (Diagram showing Cl₂ reacting with water to produce nascent oxygen).
- With Water
-
Uses of Halogens (Cl₂, Br₂, I₂)
- Chlorine (Cl₂)
- Water purification, disinfectants, bleach production, PVC manufacture, synthesis of HCl.
- Bromine (Br₂)
- Flame retardants, pesticides, pharmaceuticals, photography (AgBr).
- Iodine (I₂)
- Antiseptics (tincture of iodine), thyroid hormone synthesis, dyes, photography (AgI).
- Chlorine (Cl₂)
-
Test for Cl₂, Br₂, and I₂
- Chlorine (Cl₂)
- Pungent smell, turns moist litmus paper red then bleaches it, turns starch-iodide paper blue (displaces I₂).
- Bromine (Br₂)
- Reddish-brown vapor, turns starch paper orange-yellow, displaces I₂ from KI solution.
- Iodine (I₂)
- Violet vapor, turns starch paper blue-black (forms starch-iodine complex).
- Figure 3: Tests for Halogens (Diagram showing litmus, starch, and starch-iodide paper tests).
- Chlorine (Cl₂)
-
Comparative Study on Preparation, Properties, and Uses of Haloacids (HCl, HBr, HI)
- Preparation
- No diagrams or descriptions required as
- HCl: Prepared by heating NaCl with conc. H₂SO₄.
- HBr: Prepared by heating NaBr with conc. H₃PO₄ (H₂SO₄ oxidizes Br⁻ to Br₂).
- HI: Prepared by heating NaI with conc. H₃PO₄ (H₂SO₄ oxidizes I⁻ to I₂).
- Properties
- Reducing Strength: Increases down the group (HI > HBr > HCl). HI is strongest reducing agent due to weakest H–I bond, easily oxidized to I₂ (e.g., by HNO₃ or air). HBr moderately reducing, HCl least.
- Acidic Nature: All strong acids, acidity decreases slightly (HCl > HBr > HI) due to decreasing bond dissociation energy.
- Solubility: All highly soluble in water, forming acidic solutions. HCl forms constant boiling mixture (azeotrope) at 20.2%.
- Uses
- HCl: Cleaning metal surfaces, production of chlorides, organic synthesis, lab reagent.
- HBr: Synthesis of alkyl bromides, pharmaceuticals, analytical chemistry.
- HI: Synthesis of alkyl iodides, reducing agent in organic reactions, pharmaceuticals.
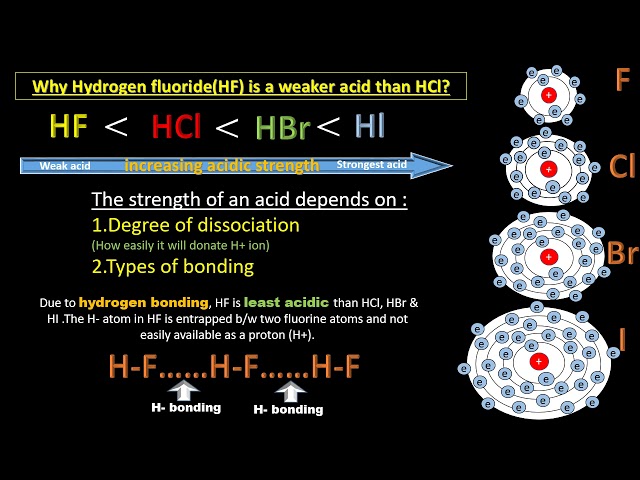
- Figure 4: Properties of Haloacids (Diagram comparing bond strength and reducing nature of HCl, HBr, HI).
- Preparation