Unit 9.2: Nitrogen (5 Teaching Hours)
-
Reason for Inertness of Nitrogen and Active Nitrogen
- Inertness of Nitrogen: Due to strong N≡N triple bond (bond energy ~945 kJ/mol), requiring high energy to break. Stable, non-reactive under normal conditions.
- Active Nitrogen: Formed by electric discharge or high-energy conditions, making it reactive. Combines with metals (e.g., Mg, Na) to form nitrides or with O₂ to form nitric oxide (NO).
-
Chemical Properties of Ammonia (NH₃)
- Action with CuSO₄ Solution: Forms deep blue complex, [Cu(NH₃)₄]²⁺: CuSO₄ + 4NH₃ → [Cu(NH₃)₄]SO₄.
- Action with Water: Weak base, forms ammonium hydroxide: NH₃ + H₂O ⇌ NH₄⁺ + OH⁻.
- Action with FeCl₃ Solution: Forms brown precipitate of Fe(OH)₃: FeCl₃ + 3NH₃ + 3H₂O → Fe(OH)₃↓ + 3NH₄Cl.
- Action with Conc. HCl: Forms white fumes of ammonium chloride: NH₃ + HCl → NH₄Cl.
- Action with Mercurous Nitrate Paper: Turns black due to formation of mercury: Hg₂(NO₃)₂ + 2NH₃ → Hg + HgNH₂NO₃ + NH₄NO₃.
- Action with O₂: Burns in presence of catalyst (Pt) to form NO: 4NH₃ + 5O₂ → 4NO + 6H₂O.
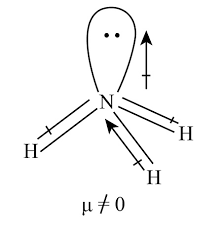
- Figure 1: Structure of Ammonia Molecule (Diagram showing trigonal pyramidal structure of NH₃).
-
Applications of Ammonia
- Fertilizer production (e.g., urea, ammonium nitrate).
- Manufacture of nitric acid (Ostwald process).
- Used in refrigeration systems due to high heat of vaporization.
- Synthesis of chemicals (e.g., explosives, dyes).
-
Harmful Effects of Ammonia
- Irritates eyes, skin, and respiratory system at high concentrations.
- Toxic if inhaled in large amounts, causing lung damage.
- Environmental pollution from ammonia leaks, contributing to acid rain.
-
Oxy-Acids of Nitrogen (Name and Formula)
- Nitrous Acid: HNO₂
- Nitric Acid: HNO₃
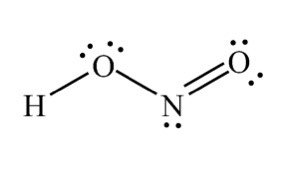
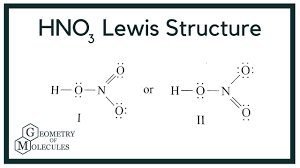
- Figure 2: Structures of HNO₂ and HNO₃ (Diagram showing molecular structures).
-
Chemical Properties of Nitric Acid (HNO₃)
- As an Acid
- Strong monobasic acid, ionizes completely: HNO₃ → H⁺ + NO₃⁻.
- Neutralizes bases to form nitrates: HNO₃ + NaOH → NaNO₃ + H₂O.
- As an Oxidizing Agent
- With Zinc: Forms different products based on HNO₃ concentration:
- Dilute HNO₃: 4Zn + 10HNO₃ → 4Zn(NO₃)₂ + NH₄NO₃ + 3H₂O.
- Conc. HNO₃: Zn + 4HNO₃ → Zn(NO₃)₂ + 2NO₂ + 2H₂O.
- With Magnesium: Similar to Zn, forms nitrates and nitrogen oxides or NH₄NO₃.
- With Iron: Passivates iron with conc. HNO₃ due to oxide layer; dilute HNO₃ produces Fe(NO₃)₂ and NO.
- With Copper: Cu + 4HNO₃(conc.) → Cu(NO₃)₂ + 2NO₂ + 2H₂O; dilute forms NO.
- With Sulphur: S + 6HNO₃ → H₂SO₄ + 6NO₂ + 2H₂O.
- With Carbon: C + 4HNO₃ → CO₂ + 4NO₂ + 2H₂O.
- With SO₂: SO₂ + 2HNO₃ → H₂SO₄ + 2NO₂.
- With H₂S: H₂S + 2HNO₃ → S + 2NO₂ + 2H₂O.
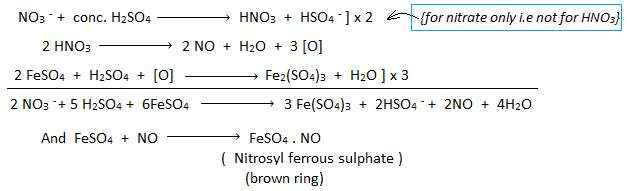
- With Zinc: Forms different products based on HNO₃ concentration:
- Figure 3: Oxidation Reactions of HNO₃ (Diagram showing HNO₃ reacting with metals/non-metals).
- As an Acid
-
Ring Test for Nitrate Ion
- Add FeSO₄ to nitrate solution, then slowly add conc. H₂SO₄ along the test tube wall.
- Brown ring forms at the interface due to [Fe(H₂O)₅NO]²⁺: NO₃⁻ + 3Fe²⁺ + 4H⁺ → NO + 3Fe³⁺ + 2H₂O.
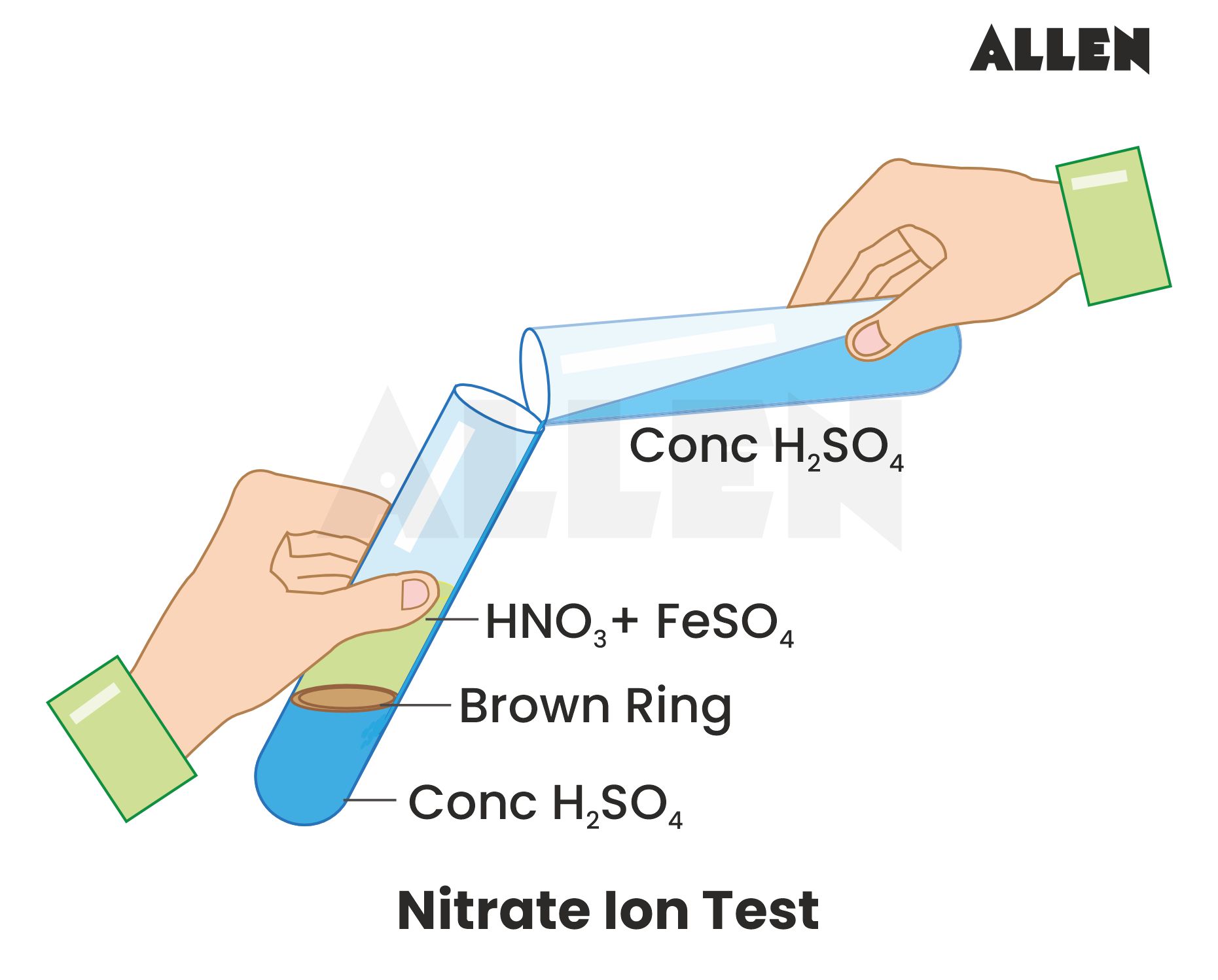
- Figure 4: Brown Ring Test (Diagram showing test tube with brown ring).