Unit 9.1: Hydrogen, Oxygen, and Ozone (4 Teaching Hours)
-
Hydrogen
- Chemistry of Atomic and Nascent Hydrogen
- Atomic Hydrogen: Formed by dissociating H₂ molecules into H atoms under high energy (e.g., electric arc). Highly reactive due to unpaired electrons.
- Nascent Hydrogen: Freshly formed H atoms during reactions (e.g., Zn + H₂SO₄ → ZnSO₄ + 2H). More reactive than H₂, reduces compounds like KMnO₄.
- Isotopes of Hydrogen and Their Uses
- Protium (¹H): Most abundant (99.98%), used in NMR spectroscopy.
- Deuterium (²H or D): Used in deuterated solvents, heavy water (D₂O), and nuclear reactors.
- Tritium (³H): Radioactive, used in nuclear fusion, self-powered lighting, and tracers.
- Chemistry of Atomic and Nascent Hydrogen

Figure 1: Structure of Hydrogen Isotopes (Diagram showing ¹H, ²H, ³H nuclear composition).
- Application of Hydrogen as Fuel
- High energy content (120–142 MJ/kg), eco-friendly (produces H₂O).
- Used in fuel cells for electricity (e.g., electric vehicles).
- Challenges: Storage and production costs.
- Heavy Water (D₂O) and Its Applications
- Contains deuterium instead of protium.
- Applications: Nuclear reactor moderator, NMR spectroscopy, biochemical tracers.
-
Oxygen
- Allotropes of Oxygen
- Allotropy: Element existing in multiple forms with different properties.
- Dioxygen (O₂): Most common, stable gas.
- Ozone (O₃): Triatomic, protects from UV radiation.
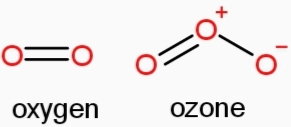
- Figure 2: Structure of Dioxygen and Ozone (Diagram showing O₂ and O₃ molecular structures).
- Types of Oxides
- Acidic Oxides: Non-metal oxides (e.g., CO₂, SO₂) form acids with water.
- Basic Oxides: Metal oxides (e.g., Na₂O, CaO) react with acids.
- Neutral Oxides: No reaction with acids/bases (e.g., CO, N₂O).
- Amphoteric Oxides: Act as acidic and basic (e.g., Al₂O₃, ZnO).
- Peroxides: Contain O₂²⁻ (e.g., H₂O₂, Na₂O₂).
- Mixed Oxides: Multiple oxidation states (e.g., Fe₃O₄, Pb₃O₄).
- Applications of Hydrogen Peroxide (H₂O₂)
- Bleaching (textiles, paper, hair).
- Disinfectant/antiseptic in medical use.
- Oxidizing agent in chemical synthesis, wastewater treatment.
- Medical and Industrial Applications of Oxygen
- Medical: Oxygen therapy, anesthesia, hyperbaric chambers.
- Industrial: Steel production, welding, chemical synthesis, rocket propulsion.
- Allotropes of Oxygen
-
Ozone
- Occurrence
- Found in stratosphere (15–35 km), formed by UV splitting O₂.
- Preparation of Ozone from Oxygen
- Pass dry O₂ through silent electric discharge: 3O₂ → 2O₃ (endothermic).
- Structure of Ozone
- Triatomic, bent molecule (116.8° bond angle), resonance hybrid.
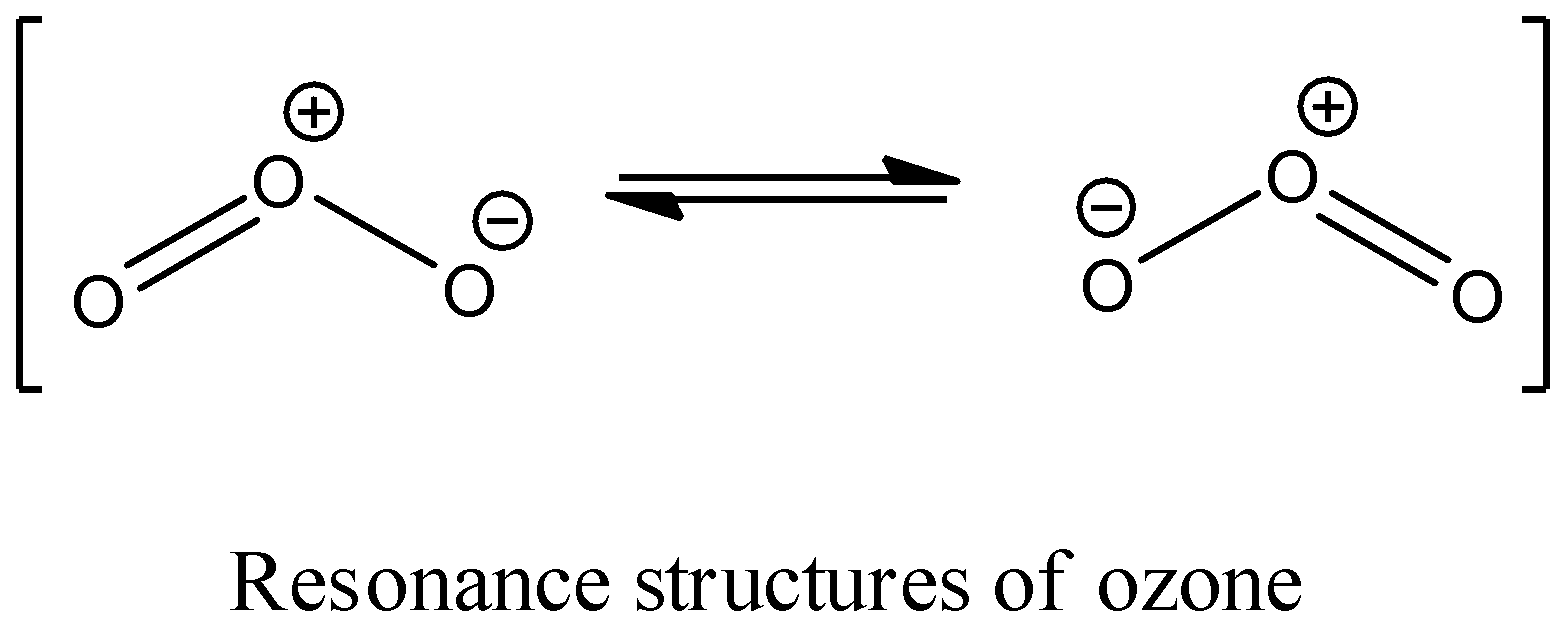
- Figure 3: Resonance Structures of Ozone (Diagram showing resonance forms).
- Test for Ozone
- Turns starch iodide paper blue (liberates I₂).
- Reduces KMnO₄ solution.
- Ozone Layer Depletion
- Causes: CFCs, halons release Cl atoms, catalyzing O₃ breakdown.
- Effects: Increased UV radiation, skin cancer, ecosystem damage.
- Control Measures: Montreal Protocol (1987), HFC alternatives, awareness.
- Uses of Ozone
- Water/air purification, bleaching, medical sterilization.
- Occurrence